Description
COVID-19 Neutralizing Antibody Rapid Test Device is available at Gentaur for next week delivery. For professional In Vitro Diagnostic Use Only
INTENDED USE:
The COVID-19 Neutralizing Antibody Rapid Test is a lateral flow chromatographic immunoassay for qualitative detection of neutralizing antibody to SARS-CoV-2 in human whole blood,serum,or plasma as an aid in the evaluation of human anti-SARS-CoV-2 neutralizing antibody titer.
SUMMARY:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped non-segmented positive-sense RNA virus. It is the cause of COVID-19, which is contagious in humans.
SARS-CoV-2 has several structural proteins including spike(S),envelope (E), membrane (M) and nucleocapsid (N). The spike protein(S) contains a receptor binding domain (RBD), which is responsible for recognizing the cell surface receptor, angiotensin converting enzyme-2 (ACE2). It is found that the RBD of the SARS-CoV-2 S protein strongly interacts with the human ACE2 receptor leading to endocytosis into the host cells of the deep lung and viral replication. Infection with the SARS-CoV-2 or SARS-CoV-2 Vaccine immunization initiates an immune response to produce antibodies which provide protection against future infections from viruses. Neutralizing antibodies block the SARS- CoV-2 spike protein bind to host ACE2 receptor-binding domain show promise therapeutically and efficiency protectively.
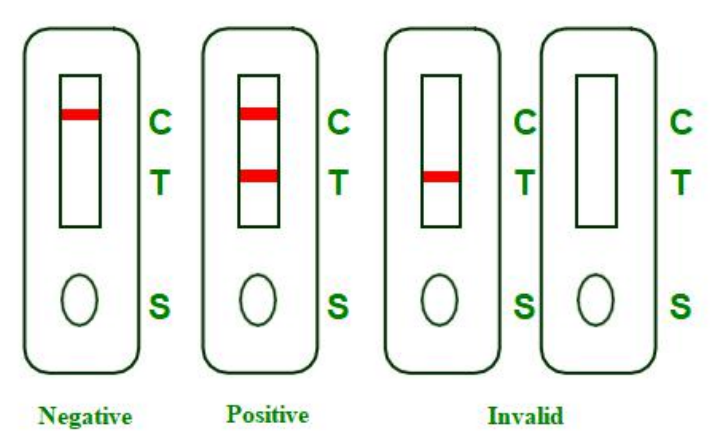
INTERPRETATION OF RESULTS
Sensitivity and Specificity:
The COVID-19 Neutralizing Antibody Rapid Test has been compared to the leading commercial COVID-19 IgG Rapid Test Device using clinical specimens from naturally infected patients.
Cross-reactivity:
The COVID-19 Neutralizing Antibody Rapid Test Device has been tested foranti-influenza A virus, anti-influenza B virus, anti-RSV, anti- Adenovirus, HBsAg, anti-Syphilis, anti-HIV, anti-rheumatoid factor, anti-M.pneumonia, anti-chlamydiapneumonia and anti-HCV positive specimens. The results showed
LIMITATION OF USE:
1. The accuracy of the test depends on the sample collection process. Improper sample collection, improper storage of samples, stale samples, or repeated freeze-thaw cycles of samples will affect the test results.
2. The test result of this kit is for clinical reference only and should not be used as the sole basis for clinical diagnosis and treatment. The clinical management of patients should be considered in combination with their symptoms/signs, medical history, other laboratory tests, and treatment responses.
3. It is recommended to review the suspicious negative results by using combined other relevant tests
Additional Information
Temperature: |
15-30°C |
Specimen: |
human whole blood, serum or plasma |
Type: |
chromatographic immunoassay |
Detection Range: |
neutralizing antibody to SARS-CoV-2 |